- About Us
- One Stop Service
- Platform
In-Vitro
Molecular Assays
Cell-Based Functional Assays
In-Vivo
Pharmacology
Cancer PharmacologyInflammation Pharmacology - Join Us
- Contact Us
- Drug Discovery
- Molecular Assays
- Cell-Based Functional Assays
- Immune Functional Assays
- Tumor Pharmacology Platform
- Inflammation Pharmacology Platform
- In-Vitro ADME/DMPK
- In-Vitro Toxicology
- Pharmacology
- Cancer Pharmacology
- Inflammation Pharmacology
- In-Vivo ADME/DMPK
- In-Vivo Toxicology
- In-Vivo Pathology
- Clinical Laboratory
Cell Therapy
The introduction of autologous or allogeneic cellular material into a patient for therapeutic reasons is referred to as cell therapy. Significant unmet needs in fields like immunotherapy and regenerative medicine are being addressed by innovative methods made possible by cellular therapy. To ensure that these new medications function consistently and efficiently, the pharmacology of cell therapies becomes very important. The principles of traditional molecular drug pharmacokinetics (PK) and pharmacodynamics (PD) can be applied to cell pharmacology to quantitatively comprehend rate-limiting restrictions on cell destiny following injection. Designing a cell therapy product to overcome any pharmacological hurdles for a specific disease application can benefit from future advances focusing on improvements in drug delivery from a PK/PD perspective.
Method
In preclinical PK-PD investigations, a drug's optimal concentration range to produce a pharmacological action is determined. In this range, there should not be any side effects, and the medication must be safe. Monitoring drug concentration is a crucial step in order to ensure both the safety and efficacy of a therapeutic regimen. Depending on the type of cell treatment and your objectives, methods can vary. For instance, two techniques—qPCR and flow cytometry—are frequently used to monitor the genetically altered T cells after adoptive transfer.
Example
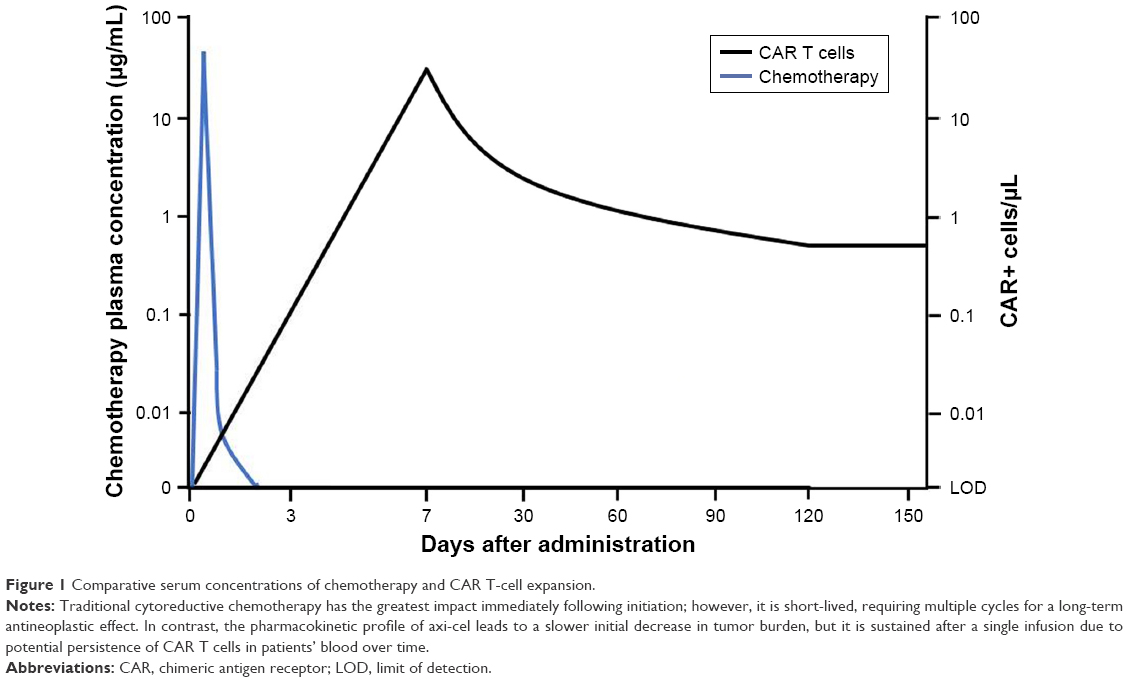
Comparative serum concentrations of chemotherapy and CAR T-cell expansion
Two-Functional-Compartments PK-PD Model in Sepsis
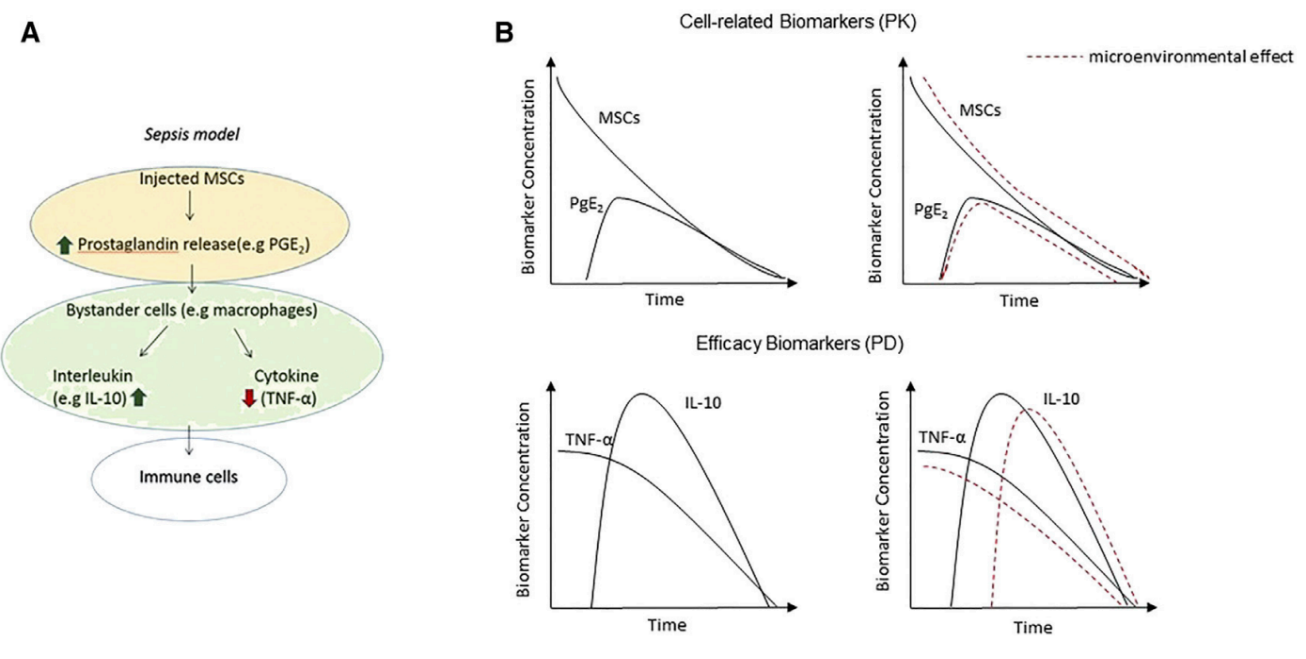
(A) Mesenchymal stromal stem cells (MSCs) are challenged in a sepsis model after i.v. delivery, causing prostaglandin-E2 (PGE2) release, which, in turn, acts on PGE2 receptors on macrophages. Macrophage receptor binding is responsible for the increase in interleukin-10 (IL-10) production and a reduction in serum tumor necrosis factor alpha (TNF-a). (B) The two-functional-compartments PK-PD model. The PK biomarkers are the MSCs and their secreted molecules leading to the PD effect. The PD biomarkers are the cytokines as markers of the therapeutic activity.
References
Jain, M. D., Bachmeier, C. A., Phuoc, V. H., & Chavez, J. C. (2018). Axicabtagene ciloleucel (KTE-C19), an anti-CD19 CAR T therapy for the treatment of relapsed/refractory aggressive B-cell non-Hodgkin's lymphoma. Therapeutics and clinical risk management, 14, 1007–1017. https://doi.org/10.2147/TCRM.S145039
Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, Hamad L, Kim S, Lacouture ME, LeBoeuf NR, Lenihan D, Onofrei C, Shannon V, Sharma R, Silk AW, Skondra D, Suarez-Almazor ME, Wang Y, Wiley K, Kaufman HL, Ernstoff MS; Society for Immunotherapy of Cancer Toxicity Management Working Group. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017 Nov 21;5(1):95. doi: 10.1186/s40425-017-0300-z. PMID: 29162153; PMCID: PMC5697162.

Shanghai Novopathway Biotechnology
Building No.5, East Huaxia Road No.333, Pudong New Area, Shanghai
BD Cooperation Email: BD@novopathway.com Tel: 021-5838 0618-501
Join Us Email: HR@novopathway.com Tel: 021-5838 0356

Beijing Sun-Novo Pharmaceutical Research
Building No.7, West Shuangying Road No.79 , Changping Area, Beijing
Website: http://www.sun-novo.com